Medical Science Publication No. 4, Volume II
PHARMACOLOGY AND TOXICOLOGY OF DRUG ADDICTION AND ALCOHOLISM*
MAJOR EDWARD C. KNOBLOCK,MSC
The problems of drug addiction among the troops and populace of a countryhas in the past led to wars between nations (the First and Second OpiumWars between England and China) and during the recent Korean incident,intelligence reports indicated that the Communists were exploiting thedrugs traffic as a means of gaining dollars to finance their operationsin Korea and elsewhere.
A full report of these operations by the Communists was prepared bythe CIC, OSI, CID and other investigating agencies of the Far East Commandfor presentation before the United Nations as evidence of Communist activityin drug traffic. Part of this evidence included the data obtained by checkingthe wholesale shipments of heroin and opium to Chinese ports; it was pointedout in this report that the wholesalers apprehended in Japan were eitherKoreans or Chinese with Communist affiliations.
One of the problems encountered by the investigating agents involvedthe apprehension of the narcotic users who often resorted to very cleverruses to conceal the sources of their supply. Even though Special Regulationsand Administrative Memoranda amply prescribed the prohibition against thepossession of narcotic substances and implements used in the administrationof such substances, the user of narcotics quickly became aware of theseregulations and made it a point to get his drugs directly from the "pushers"who were usually found in the gay quarters of Japanese and Korean cities.It became increasingly difficult for investigating agents to apprehendthe users in possession of drugs or implements for injection. Consequently,the sale of narcotics continued at a rate consistent with the number ofsusceptible troops within an area and was concentrated within those citiesin Japan and Korea which were centers for troop and harbor activities.
The CID laboratory and the 406th Medical General Laboratory in Tokyowere the organizations whose facilities supported the investigating agents;in 1952 the resources of the 1st Medical Field Labora-
*Presented 27 April 1954, to the Course on Recent Advances in Medicine and Surgery, Army Medical Service Graduate School, Walter Reed Army Medical Center, Washington, D. C.
171
tory in Korea were added to this service. Close liaison was maintainedbetween these laboratories, and this sharing of information was found tobe mutually valuable when cases involving the services of both laboratorieswere being considered. The medical laboratories were charged with the analysesof blood and urine specimens taken from suspected drug users and for thetoxicologic examinations of autopsy material; the CID laboratory identifiedsamples of suspected narcotics that were brought in by the field investigators.With such an arrangement, the interlaboratory sharing of information wasa necessity.
The drugs most commonly identified in the laboratories were heroin,morphine, opium, various types of barbiturates, amphetamines, marihuana,and a wide variety of alcoholic beverages. The bulk of the toxicologicexaminations performed by the medical laboratory involved the search forthese substances. All the autopsies in which the examining pathologistcould not determine the cause of death were followed by toxicologic analyses.Those specimens collected from the remains of suspected drug addicts werealso submitted to the medical laboratory, all of which required considerablework in order to insure that the investigating authorities received thefullest support. These investigations led to an improved system of identifyingthe types of opiates whose description will be included in this report.
Aside from assuring proper analytical technics the practical considerationsin establishing an operational toxicology laboratory included a procedurewhich assured the adequate sampling of all necessary specimens and testsfor carrying out the examinations requested, an adequate procedural protocoland description of the conditions surrounding the circumstances of death,as well as a system providing that the specimens be subjected to a legallyresponsible chain of custody from the time of collection until the completionof the examination. Without fulfilling this latter requirement the performanceof a toxicologic examination is valueless since the report itself cannotbe considered as adequate evidence in the courts. Direct transportationof specimens to the testing laboratory by a trustworthy courier withoutrecourse to intermediate channels provides a satisfactory link in the chainof custody from examining physician to the laboratory. Complete examinationof the specimen container and a satisfactory seal are also necessary. Shouldcourier service not be available, the use of registered mail addressedto the attention of the toxicologist is also satisfactory. The generalrequirements for submitting specimens for toxicologic examination as outlinedby TB Med 237, Department of the Army Technical Bulletin, satisfactorilycover the general aspects of the sampling procedure and transfer of samples.
172
During the years 1950, 1951, and 1952 the various examinations by the406th Medical General Laboratory in regard to drugs and alcohol are summarizedin the following table:
1950 | 1951 | 1952 | |
Total autopsy cases examined | 171 | 201 | 215 |
Narcotics findings: | 1 | 18 | 13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total clinical cases | ----- | 404 | 618 |
Narcotics findings: | ----- | 102 | 67 |
Barbiturates | ----- | 85 | 55 |
The toxicology and pharmacology of each of the drugs encountered willbe discussed as related groups.
Toxicology of Alcoholism
The detection of alcohol consumption presented the analytical laboratorywith its primary forensic problem since automobile accidents, accidentaldrownings, fights, and miscellaneous disturbances associated with intoxicationwere not infrequent. Alcohol taken by mouth is partly absorbed by the stomachbut primarily by the small intestine. Foods high in the amino-acids, glycineand alanine lower the rate of food absorption (1)-a reaction similarto that which occurs when foods and aqueous beverages are diluted withalcohol. Alcohol distributes itself into tissues and secretions by diffusion.Those tissues with the highest water content are found to contain the highestconcentration of alcohol. Approximately 90 percent of the ingested alcoholis metabolized within the body with 2 percent being expired through thelungs and 8 percent eliminated by the kidney
(2). Maximum blood concentrations in man occur within 10 to 20 minutesafter an intravenous injection of alcohol, but it takes 25 to 35 minutesfor the same dose to reach maximum alcohol concentrations in the cerebrospinalfluid (3). The rate at which the blood-alcohol concentration dropsfrom the maximum level, within certain limits, is linear and appears tobe independent of the amount injected (4).
Alcohol is a normal constituent of the body although its life in tissuesis short and the concentration is small (5). It is normally oxidizedin the body by an enzyme system using alcohol which is primarily foundin the liver. Mirsky and Nelson (6) have shown that liver damageretards the oxidation of alcohol and that the liver oxidizes 90 percentof the absorbed alcohol.
173
Ethanol is normally oxidized to form acetaldehyde which upon furtheroxidation is converted to acetic acid that may then enter the tricarboxcylicacid cycle where the oxidation is completed with the formation of carbondioxide and water. If some chemical such as antabuse (tetraethyl thiuramdisulfide)or another enzyme inhibiting agent interfered with the cycle involvingthe oxidation of the alcohol, the intermediate products will accumulateand produce their own effect. Habituation apparently does not acceleratethe metabolism of alcohol which continues at a relatively consistent rateof 10 cc. per hour.
Ethanol acts upon the sensory areas of the cortex and extends to themotor areas as the blood concentration increases. The first effect is theso-called "social stage" which is the depression of the inhibitorycenters of the cortex. It is this observed effect that prompts some personsto believe alcohol to be a stimulant even though its pharmacologic effectis always that of a depressant. The conditioned reflexes are slowed andcoordination impaired under the influence of alcohol. Many attempts havebeen made to correlate the activity of the individual with the blood alcohollevel and the mean of these attempts would approximate the following:
Blood alcohol | Reaction |
0-55 mg./100 ml. blood | Normal |
55-100 mg./100 ml. blood | Querulous, false euphoria |
100-250 mg./100 ml. blood | Irregular gait, lack of coordination, mental confusion |
250-500 mg./100 ml. blood | Respiratory depression, stupor, possible death |
The effect of alcohol on the cortex reaches its peak while the bloodalcohol level is increasing. During this time the cortex cells are adjustingto the effects of the alcohol. Tolerance may be increased by repeated dosesas shown by the response of the habitual drinker compared with that ofthe uninitiated.
Alcohol in large quantities affects not only the cortex but continuesto the medulla as well as exerting a strong depressant action on the respiratorycenters. Deaths due to acute alcoholic poisoning are the result of respiratoryparalysis (7).
The technics employed to detect the presence of alcohol in the bloodare reliable; most of these methods depend upon the oxidation of alcoholby a standard potassium dichromate solution. The chromous ion formed maybe measured or the residual dichromate may be titrated with a standardthiosulfate solution.
Alcoholic beverages include the wines (12 percent), fortified wines(25 percent), beer (2.0-6 percent), whiskeys (45-55 percent), and variousforms of distilled wines (cognac, brandy, etc.). These drinks
174
produce intoxication dependent upon their alcoholic content, but theincreasingly unfavorable after-effects that result from mixing differenttypes of liquors may possibly be the result of synergistic action betweenthe various impurities in the liquors (8). Little is known aboutthis particular phenomenon, however.
Methyl alcohol (methanol) oxidizes much more slowly in the body thandoes ethanol and its oxidation produces formaldehyde and formic acid whichare extremely toxic. Methanol is also a depressant of the central nervoussystem; it evokes, besides, cerebral edema, neuritis and a specific toxicityfor the ganglionic cells of the retina with the resultant blindness observedin methanol poisoning. Methanol also is hepatotoxic and may produce fattydegeneration of the liver (9).
The beers and liquors examined in Japan during the 3-year period 1950-1952(inclusive) showed no sample to be outside the limits allowed for medicinalbeverages, as specified by the United States Pharmacopoeia, even thoughJapanese law allows 0.2 percent methanol to be present in distilled beverages.The same cannot be said for those brandies and other beverages that wereobtained in Korea, the composition of which ranged from mixtures of chloroformand water, ethylene glycol, pure methanol, to methanol-containing brandies.These liquors were all attractively packaged but constituted a real threatto the life of the consumer-if he had the fortitude to swallow them. Deathsrecorded herein as due to methanol poisoning occurred exclusively in Koreaalthough the consumption of methanol in one drinking party aboard a shipaccounted for six of the deaths due to methanol poisoning in 1951.
Toxic Effects Produced by Use of Barbiturates
Drugs used for their sedative and hypnotic effect are often broughtto the attention of the laboratory as a result of accidental overdosageor as a result of suicides. Usually these drugs are classed under fourheadings (long, intermediate, short, and ultrashort) depending on theirduration of action upon the cerebral cortex and thalamus. The durationof depression and the intensity of action depends upon the structure ofthe drug, the amount used and the mode of administration. The various classesof the barbiturates are derived from barbituric acid (malonylurea) andderive their individual properties from the various attached prostheticgroups all of which cover a wide range of organic structures; the substitutionsusually being made in the 5, 51, and 1 positions.
The barbiturates affect the sensory and motor areas of the brain, whichis the basis for their physiologic action in producing sleep and alleviatingconvulsions. Therapeutic doses of barbiturates produce only slight respiratorychanges but large doses markedly depress respi-
175
ration. When death is caused by these drugs, it is the result of respiratoryfailure or of the complications associated with a decreased rate of respiration.Therapeutic doses do not affect the circulation, but large doses produceperipheral vasodilation and a fall in blood pressure. With removal of thedrug, the blood pressure returns to normal.
Barbiturate poisoning is characterized by shallow and slow breathing.Mental confusion, ataxia, clammy skin, reduced reflexes and constrictedpupils are noted in early stages of this type of poisoning. As the dosageis increased, a fall in body temperature, dilation of the pupils and comaare noted. Severe poisoning usually results from an intake that rangesbetween 5 and 10 times the therapeutic dose. Accidental poisonings haveoccurred when persons who were accustomed

to the drug took repeated doses during a period of blurred consciousnessin order to induce sleep. Various psychogenic factors, usually associatedwith insomnia, are largely responsible for habituation to the barbituratessince sleep is the most common effect these drugs produce. Although theremay be a certain degree of transient tolerance in man, possibly motivatedby a strong psychogenic factor (10) which may result in a form ofhabituation, withdrawal symptoms such as occur in the alcoholic and opiateaddict are not generally shown with barbiturate abstinence.
Toxicologic search for the presence of barbiturates is most successfulwhen the urine is analyzed since the slow- and intermediate-acting barbituratesare largely eliminated by this route. The short- and ultrashort-actingdrugs are largely detoxified by the liver (11) and are
176
not eliminated in the urine. For this reason it is very important thatthe toxicologist be apprised of the type of drug the patient or deceasedis or was suspected of using. At autopsy the brain is the organ of choicefor examination. Chemical methods of examination are very reliable andthe method of Goldbaum (12) using the ultraviolet spectrophotometerwas found most adaptable to the work done in the Far East Command.
Deaths due to barbiturates were associated with suicides, accidentaldrowning, concurrent administration with heroin, carbon monoxide asphyxiationfrom space heaters in hotels, and other conditions where sedation preventedthe response of the individual to a danger stimulus.
Marihuana-Its Source, Physiological Effect and Detection
The drug prepared from the flowering tops of Cannabis satira wasbrought to the attention of the 406th Medical General Laboratory shortlyafter United Nations troops entered Korea. The Koreans cultivate this plantfrom the hemp fiber which forms a large part of domestic fabrics. The readyaccess of these plants allowed free traffic for the marihuana smoker. Severalsamples of this drug were submitted for laboratory identification but thelaboratory was never apprised of any widespread use of this narcotic.
Cannabis (known popularly as marihuana, hashish, and bhang) is producedas a narcotic by removing the flowering tops of this hemp plant beforethe fruits have developed. At this time the highest concentration of thenatural resin is to be found in the plant. The active agents which havebeen isolated from cannabis are an alcohol, cannabinol, and a volatileoil. The alkaloids of cannabis are apparently not pharmacologically active.
Cannabinol is highly toxic and has a strong narcotic effect manifestedby a perversion of time, distance and sound perceptions. It produces differenteffects in different personality types. Some are offered a complete escapefrom reality, sensuous dreams are experienced by others, and in othersthere is a complete release of inhibitions. Habitual doses are likely toresult in facial bloating, ataxia, moral degeneration and mental stupor.For the occasional smoker the mental effects are the most pronounced andperipheral effects are negligible.
Smoking of the dried plant is the most common route of administrationand the habitué usually desires company in his venture. Apparentlyno added physiologic tolerance to the narcotic develops with continueduse and only the psychic factors appear important upon withdrawal of thenarcotic from the user (13).
The most satisfactory method used to identify marihuana is the microscopicexamination of suspected cigarettes. The characteristic
177
spiculated stem of cannabis is easily identified by the botanist. Chemicaltests have been described but were not used for laboratory examinationssince a qualified botanist was available.
Amphetamines-Their Possible Toxicologic Effects
Various types of stimulants were often brought into the laboratory.In Japan these are generally produced in 1 ml. or 2 ml. sterile ampulescontaining 10 mg. amphetamine for injection which are marked under a varietyof names such as "Methylpropamin," "Agotin," "Neoagotin,""Zedrin," "Hospitan," "Metabolin," "Koripron,"and a variety of other names. These preparations were readily availablefrom the Japanese pharmacies and some instances of habitual use were reportedamong the United Nations personnel.
Amphetamine, 1-phenyl-2-aminopropane, is most commonly called by itstrade name Benzedrine. Its high volatility makes it most useful as a vasoconstrictorwhen inhaled through special vaporizers (inhalers). Taken parenterallyor orally, the amphetamine derivatives stimulate the central nervous systemand are useful in treating acute effects of barbiturates or opiates bystimulating brain circulation which assists in removal of the depressingdrug. This stimulant action produces in many persons a mild euphoria, brighterspirits and loss of fatigue; it was sought in Japan by the students andworkers who spent many tiresome hours daily at a job. However, the basicneed of the body for rest is in no way affected by the use of this stimulantand its prolonged use can lead to a general weakening of the body.
As evidenced by the manner in which amphetamine is often packaged, thefavorite route-also the most effective (14)-is the subcutaneousinjection of the drug. Danger from continued dosage of amphetamine liesin the development of hypertension, gastrointestinal disturbances, vexationand restiveness. Toxic doses may show dilation of the pupils, palpitation,chills and collapse which respond to treatment by the short-acting barbiturates.
The drug may become habit-forming with the development of psychic dependence.In recent years the use of Benzedrine inhalers and similar non-prescriptionitems has been largely replaced in the United States by Benzedrex whichis N-Methyl-ß-cyclohexylisopropylamine. Benzedrex retains the nasalconstrictive properties but lacks the stimulating properties for the centralnervous system shown by Benzedrine.
Although amphetamine was readily available and its misuse occasionallyreported to the laboratory, no widespread use of this substance among UnitedNations personnel was reported. No acute or fatal cases were reported tothe laboratory.
178
Alkaloids
Description of Several Alkaloids
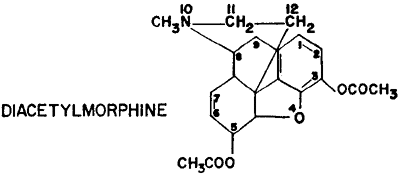
Opiates were the class of drugs about which the laboratory wasmost deeply concerned. The detection and analysis of these narcotics requiredmore work than was needed to find and study the other drugs of interestto the Army toxicologist. The primary opiate found on the drug market washeroin (diacetylmorphine) which has the strongest addicting propertiesof the opiates. As stated in the early paragraphs of this report, the drugwas readily available to the addict, both in Japan and Korea, and investigatingagents were often stymied in their work by not being able to catch thesuspect in possession of the drug or the implements used to administerit. Since the opiates are in part excreted in the urine as free or conjugatedderivatives of alkaloids, produced by the body's detoxication processes,laboratory personnel were assigned the task of developing a satisfactoryprocedure for the identification and isolation of opiates found in urinesamples taken from suspects. A satisfactory and reliable chromatographicpurification procedure as well as an adaptation of a hydrolytic processfor urinary extraction were developed and are to be described in this report.
Opium, the sun-dried latex of the unripe fruit of Papaversomniferum, has a total alkaloid content approximating 20 percent.For economic reasons, mainly cheap labor, the opium poppy is grown in theOrient, but this plant will grow equally well in the United States.
Two principal alkaloid types may be derived from opium-(1) thephenanthrene derivatives include morphine, codeine and thebaine which actprimarily on the central nervous system, and (2) the 1-benzylisoquinolinederivatives, which include narcotine and papaverine (the more commonlymentioned of this class alkaloids) that exert little effect on the centralnervous system. A total of 23 alkaloids have been isolated from opium butthe most important are those that have been named.
Morphine is the main opium alkaloid, composing approximately9 percent of opium. Although the structure of morphine has been definitelyestablished, attempts to synthesize the drug have not been successful.Morphine contains the phenanthrene nucleus with a phenolic hydroxyl groupand a secondary alcohol hydroxyl group which are subject to alkylationor esterification with numerous organic radicals. Oxidation and reductionof the secondary alcohol produces additional derivatives which have beenstudied extensively (15). This structural variation of morphinehas recently produced an excellent morphine antagonist, N-allyl morphine(Nalline) which is specific in counteracting the analgesic and depressanteffect of morphine (16).
179
The tertiary nitrogen atom of morphine gives the molecule basic propertieswhich permit it to be readily converted to such salts as the sulfate, hydrochloride,etc.
Morphine acts primarily on the medullary centers of the brain as evidencedby the depression of the respiratory, cough and vasomotor centers as wellas the stimulation of the vomiting centers. When death occurs as a resultof acute morphinism, respiratory failure is the primary cause of death.This was the principal clue in a number of deaths suspected to have beencaused by the administration of dope. The principal pharmacologic responseby the body to this drug is the relief of pain, but in some individualsit elicits a feeling of euphoria and may produce sleep. Morphine has strongaddicting power and produces violent withdrawal symptoms in many of itsaddicts.

Diagnosis of the addict is by physical examination of the body withemphasis on such evidence as the presence of needle marks over the largeblood vessels, pupillary constriction, dulling of sensory response, lethargy,drowsiness along with a record of association with narcotics users or findingthe victim in an area where these drugs are sold. These findings justifystrong suspicion of an opiate overdosage.
Heroin, a synthetic drug, is diacetylmorphine and is more stronglyaddicting than morphine. It readily produces euphoria in many individualsbut is also approximately five times as toxic as morphine. Its manufactureis forbidden by law in the United States but it is not difficult to produce.Korean dope peddlers were producing high-grade heroin, 90 to 95 percentpure, from opium, using small alcohol burners and reaction flasks whichcould all be assembled in a box the size of an orange crate. Heroin trafficwas found to be particularly vicious when analysis of samples showed theactive drug content to range from 5 to 99 percent. Among the many adulterantswith which this opiate was prepared, sugar, starch, bicarbonate of soda,sand and
180
ground rice husks were commonly used. Because the manufacture of thedrug was not standardized, each sample most likely contained one of a widevariety of possible dosages (normal dose is 4 to 10 mg.). When coupledwith the fact that a harmful adulterant could be used, this meant thatthe addict was flirting with death with each injection. The principal methodof drug administration was by hypodermic needle, although heroin and opiumwere smoked in some cases and taken orally in others.
Heroin by deacetylation in the body is converted to morphine and followsthe pattern of morphine metabolism. The principal substances recoverablefrom the urine are conjugated products which may be obtained by the useof suitable hydrolytic procedures.
Codeine composes approximately 0.3 percent of opium. Structurallyit is methyl morphine. This alkaloid is obtained principally by the syntheticprocess of morphine methylation. Codeine is widely used in medicine andis less stringent than morphine in its action on the medullary centers.It is likewise much less addicting. Codeine has approximately one-thirdthe activity of morphine on the cough center and approximately one-twentiethof morphine's activity on the higher centers of the brain. This propertyleads to wide prescription of codeine in cough syrups and complicates thework of the toxicologist since morphine and codeine react similarly withnumerous test re-

181
agents. However, there are sufficient specific reactions, as will bedescribed in this report, to permit identification of either alkaloid.
Laboratory Analysis
The following is the process for isolating and identifying the alkaloidsas developed by personnel of the 406th Medical General Laboratory:
Alkaloids. With few exceptions, every toxicologic specimen receivedis routinely examined for the presence of these narcotics. A new methodfor the extraction of alkaloids from body fluids has been developed andthe results gained in hundreds of extractions have shown the method tobe superior to any used in the past.
Detecting the Presence of Alkaloids by the Analysis of Urine Samples.Gross and Thompson (17) have shown that acid hydrolysis willpermit the extraction of that portion of morphine from urine present ina conjugated form. An amount of 50 to 100 ml. is placed in a 500 ml. Erlenmeyerflask and subjected to hydrolysis by the addition of concentrated (10 percentby volume) hydrochloric acid and by autoclaving the sample at 15 poundspressure for 30 minutes. The specimen is cooled, transferred to a 250 ml.centrifuge bottle where 25 to 50 ml. of chloroform is added. The bottleis stoppered, shaken for 3 minutes and centrifuged for about 15 minutesat 3,000 rpm in an International size II centrifuge. With the aid of a50 ml. volumetric pipette and rubber bulb, as much of the chloroform layeras possible is removed and filtered through filter paper. The extractionis repeated a second time and the chloroform is combined and evaporatedin a small beaker. The residue is examined for barbiturates and other acidand neutral substances.
Experience has shown that hydrolysis produces a dark brown discolorationin many of the normal constituents of urine. This material is extractablewith chloroform from an acid solution so that the residue in most casesis not entirely suitable for examination. Where the presence of barbituratesis suspected, a portion of urine is extracted without hydrolysis and thisresidue is examined for that type of preparation. The aqueous solutionremaining in the centrifuge bottle is made alkaline by adding 10 percentsodium hydroxide solution and the extraction is repeated with chloroform,in two portions. The residue will contain alkaloids other than morphine.For the extraction of morphine, the aqueous solution is first made acidwith hydrochloric acid and then basic with ammonium hydroxide solution.Two extractions are made with a 9:1 chloroform-alcohol mixture.
Paper Chromatography of the Opium Alkaloids. The difficultiesattending the purification and identification of opium alkaloids in tissuesand body fluids have long plagued the toxicologist. The amounts of alkaloidspresent in toxicologic specimens are extremely
182
small and must be isolated in a relatively high state of purity beforechemical identifications can be made. Because the isolation technics areimperfect and the chemical tests subtle, the experience and skill of thetoxicologist is often the deciding factor between a positive and negativealkaloid identification. Since the finding of narcotic alkaloids raisesquestions of grave nature, there is a reluctance to report as positiveany but the most conclusive chemical tests; doubtful or borderline reactionsare reported as negative. The application of the paper chromatography technichas done much to minimize these difficulties.
The general methods and principles of paper partition chromatographycan be described briefly as follows: A small drop containing the soluteunder investigation is placed near one edge of a strip or sheet of filterpaper. After the spot has dried, the paper is placed in a sealed container.The atmosphere within the chamber consists of air saturated with waterand the vapors from an appropriate solvent. After the paper has come toequilibrium with the atmosphere, the end of the paper containing the spotis brought into contact with the solvent. The solvent flows past the spotby capillary action and up the length of the paper. By this time the solutewill have moved a distance along the paper, the amount of this movementdepends upon the relative solubility of the solute in water and in thesolvent (i. e., the partition coefficient). The movement of the solutezone has been explained conveniently as follows: "The cellulose fibershave a strong affinity for the water present in the solvent phase but verylittle for the organic liquid. The paper itself is thought of as an inertsupport holding a stationary aqueous phase. As solvent flows through asection of the paper containing the solute a partition of this compoundoccurs between the mobile organic phase and the stationary water phase.Thus, some of the solute leaves the paper and enters the organic phase.When the mobile liquid reaches a section of the paper containing no solute,partition again occurs. This time, solute is transferred from the organicphase to the paper phase. With continuous flow of solvent, the effect ofthis partition between the two phases is the transfer of a solute fromthe point of its application on the paper to a point some distance alongthe paper in the direction of solvent flow (18)."
General Chromatographic Procedure. Rectangular sheets of filterpaper measuring 28 x 32 cm. are cut from larger sheets of No. 1 Whatmanpaper. A pencil line (base line) is drawn 4 cm. from the longer edge ofthe paper, paralleling that edge. On points along this line, at 3 cm. intervals,0.005 to 0.01 ml. drops of the samples to be chromatographed are placedusing 0.1 ml. Mohr pipettes. The paper is dried at room temperature. Whenvolumes greater than 0.01 ml. are to be chromatographed, the solution isapplied to the paper in aliquot portions allowing each aliquot to dry beforefurther additions are
183
FIGURE1. Chromatographic apparatus.
made. The paper is then bent perpendicularly to the base line into acylinder and fastened at the end opposite the base line with paper clipsusing a paper bridge to separate the edges which close the cylinder. Thepaper cylinder is lowered, base line end downward, into a Smillie jar (fig.1). The jar is clamped shut, using stopcock grease to effect an air-tightseal. Employing a pipette, the aqueous phase of the two-phase, water-solventmixture is introduced through opening A onto cotton surrounding deliverytube B. This arrangement fa-
184
cilitates saturation of the atmosphere. Enough solution is applied tosaturate the cotton, any excess falling into beaker C. Opening A is stoppedand the apparatus is untouched for a period of 4 or 5 hours during whichtime the moisture content of the paper reaches equilibrium with the inclosedatmosphere. At the end of this period, 50 ml. of the solvent phase of thetwo-phase mixture is introduced into the bottom of the jar via funnel Dand delivery tube B. The solvent immediately begins its capillary climbup the paper. After approximately 13 hours, the solvent front has advancedto within 2 or 3 cm. of the top of the paper cylinder.
The paper is then removed and laid flat and the solvent front boundaryis marked immediately with pencil. The paper is air dried at room temperatureby clipping to an electric fan. Using an atomizer, the paper is sprayedwith a reagent to show the location of the alkaloid areas. In establishingthe Rf value, a point is selected in the alkaloid spot wherethe greatest color density is observed. The distance between this pointand the base line divided by the distance between the base line and thesolvent front boundary is the Rf value of that spot.
Paper Chromatography of Pure Opium Alkaloids. Before toxicologystudies could be initiated, it was necessary to establish a workable procedureusing pure alkaloids. While more than 25 alkaloids have been isolated fromopium, about 98 percent of the total alkaloid content of opium is madeup of 6 alkaloids-namely, morphine, narcotine, papaverine, thebaine, codeine,and narceine. Accordingly, of the naturally occurring opium alkaloids,only these 6 compounds were considered in this study. In addition, 3 syntheticmorphine derivatives were included in the series-heroin, donine, and apomorphine.
Stock Solutions. Solutions of the 9 alkaloids and their hydrochloricand acetic acid salts were prepared. The free bases were made up as 0.5percent solutions and the salts in concentrations equivalent to 0.5 percentsolutions of their corresponding free bases. Fifty percent ethanol wasemployed as the solvent for all of the alkaloid salts except narcotineacetate, which was prepared with 95 percent ethanol. Of the free bases,narcotine, thebaine, heroin, and apomorphine were dissolved in chloroform;papaverine, codeine, and dionine were prepared with 50 percent ethanolsolutions; morphine was made up with isoamyl alcohol; and narceine with10 percent ammonium hydroxide.
Chromatographic Developing Solvents. The following solvents wereemployed experimentally in an attempt to find a suitable chromatographicdeveloping agent:
n-butanol
n-butanol, acetic acid
n-butanol, ammonium hydroxide
185
n-butanol, chloroform
n-butanol, chloroform, acetic acid
n-butanol, chloroform, ammonium hydroxide
n-butanol, saturated sodium bicarbonate
n-butanol, phosphate buffer (serial pHs from 4.5 to 8.5)
n-butanol, hydrochloric acid
n-butanol, sodium hydroxide
isoamyl alcohol
isoamyl alcohol, acetic acid
isoamyl alcohol, ammonium hydroxide
isoamyl alcohol, pyridine
butyl acetate
butyl acetate, acetic acid
butyl acetate, ammonium hydroxide
toluene
toluene, acetic acid
toluene, ammonium hydroxide
chloroform
chloroform, acetic acid
chloroform, ammonium hydroxide
pyridine
aniline
ethanol (various concentrations), acetic acid
ethanol (various concentrations), ammonium hydroxide
ethylene glycol monoethyl ether
Of these, only butanol-acetic acid and isoamyl alcohol-acetic acid gavewell-positioned, clear-cut separations. Because its capillary climb upthe paper is slower and therefore more suitable for laboratory time arrangements,the isoamyl alcohol solvent was selected in preference to the butanol solvent.It is prepared as follows: isoamyl alcohol (100 ml.), glacial acetic acid(10 ml.) and water (50 ml.) are shaken together thoroughly in a separatoryfunnel and the mixture allowed to stand for 3 days at room temperaturebefore being separated into its solvent and aqueous phases. The 3-day standingperiod enables the constituent solutions to reach an equilibrium with respectto the formation of isoamyl acetate.
Spot Developing Reagents. Preliminary trials with a number ofreagents giving color reactions with opium alkaloids led to the selectionof a modified Dragendorff's reagent and a potassium iodoplatinate solutionas the reagents most nearly filling the requirements of this study. Theyare prepared in the following manner:
Dragendorff's Reagent. A mixture of bismuth subnitrate (2.5 gm.),water 20 ml.), glacial acetic acid (5 ml.), and potassium iodide (4 gm.-previouslydissolved in 10 ml. of water) are mixed. The precipitate that forms onstanding for about 3 hours is removed by filtration. The filtrate is thestock solution. Just before using, one part of the stock solution is mixedwith two parts of glacial acetic acid and three parts of water.
186
Potassium Iodoplatinate Reagent. 1 ml. of 10 percent platinicchloride and 25 ml. of 4 percent potassium iodide are mixed and enoughwater added to make the volume of the resulting solution equal 50 ml.
The sensitivities of these two reagents were tested against 10, 20 and50 microgram spots of the 9 alkaloids which had been developed chromatographicallyas described above. All quantities of each of the alkaloids were appliedto the paper in 0.01 ml. volumes. The results are shown in table 1.
Table 1. Reagent Sensitivity for Alkaloids
|
| Quantity of Alkaloid | |||||
10 mu | 20 mu | 50 mu | ||||
D 1 | P 2 | D | P | D | P | |
Morphine | Minus | Plus | Minus | Plus | Plus | Plus. |
Narcotine | Plus | Minus | Plus | Plus | Plus | Plus. |
Papaverine | Plus | Plus | Plus | Plus | Plus | Plus. |
Thebaine | Plus | Plus | Plus | Plus | Plus | Plus. |
Codeine | Plus | Plus | Plus | Plus | Plus | Plus. |
Narceine | Minus | Minus | Minus | Minus | Plus | Plus. |
Heroin | Plus | Plus | Plus | Plus | Plus | Plus. |
Dionine | Plus | Plus | Plus | Plus | Plus | Plus. |
Apomorphine | Minus | Plus | Plus | Plus | Plus | Plus. |
1Dragendorff's reagent.
2Potassium iodoplatinate reagent.
It is seen from the table that when potassium iodoplatinate was usedall of the alkaloids but narcotine and narceine could be detected in 10microgram quantities, and that the narcotine spot was visible at the 20microgram level. With Dragendorff's reagent, morphine, narceine, and apomorphinecould not be discerned at a 10 microgram level. Fifty microgram spots ofall alkaloids were visible when either reagent was used. The platinateis the reagent of choice because it offers a better contrast between thespot and its background and is more sensitive in its reaction with morphine,the alkaloid of greatest interest to the personnel of the 406th MedicalGeneral Laboratory.
Rf Values. The nine opium alkaloids were chromatographedin the form of free bases, acetates, and hydrochlorides. More variationwas observed with the alkaloid salts than with the free bases. The resultsobtained with the latter, using both the butanol-acetic acid solvent andthe isoamyl alcohol-acetic acid solvent are reported in table 2.
187
Table 2. Comparison of Solvents for Alkaloids
Alkaloid | Butanol-acetic acid solvent Rf (A) | Isoamyl alcohol acetic acid solvent Rf (B) | B/A |
Morphine | 0.41 | 0.12 | 0.29 |
Narcotine | 0.76 | 0.62 | 0.82 |
Papaverine | 0.74 | 0.61 | 0.82 |
Thebaine | 0.65 | 0.43 | 0.66 |
Codeine | 0.48 | 0.20 | 0.42 |
Narceine | 0.67 | 0.46 | 0.69 |
Heroin | 0.61 | 0.36 | 0.59 |
Dionine | 0.57 | 0.30 | 0.53 |
Apomorphine | 0.65 | 0.33 | 0.51 |
The spot patterns obtained with both solvents are similar, the generaldifference being the lower Rf values produced with isoamyl alcohol.That this lowering of Rf values is not proportionate throughoutis seen by noting the B/A ratios given in the table. Morphine and codeinemigrate at less than the average retarded rate. The reverse is true ofnarcotine and papaverine.
The values given for the butanol solvent represent averages of fivedeterminations, three of which were run simultaneously, but in differentchambers. The average variation between the Rf values of thealkaloids of the two nonsimultaneous trials is 7.2 (0.9 to 15.8) percent.In the simultaneous trials the deviation from the mean never exceeded 3.2percent and the average is only 1.9 percent. Variation is even less ifone considers the relative position of the spots, i.e., the relation ofeach alkaloid spot to all others on the same sheet. Such a considerationcan be appreciated mathematically by comparing the Rf of eachspot with the average Rf of all spots. Here, the deviation fromthe mean does not exceed 1.9 percent (average: 1.2 percent).
The values given for the isoamyl alcohol solvent were obtained froma single trial. Experience has shown that there is considerable variationin the Rf values obtained over a period of months. The followingfactors are known to influence Rf values: (1) the paper employed,(2) temperature, (3) quantity of material being chromatographed, (4) extraneoussubstances, (5) degree of saturation of the paper with water and solvent,(6) starting point with relation to solvent, (7) height of chromatograph,and (8) volume of starting spot. In these experiments temperature was theonly factor not controlled. Indeed, it was found in many subsequent determinationswhere morphine, codeine, and heroin were employed as standards for toxicologicdeterminations that temperature had a definite influence on the Rf
188
values obtained. The values reflected the effects of seasonal room temperaturechanges, higher temperatures giving higher Rf values. A summaryof 92 trials employing the isoamyl solvent against morphine, codeine, andheroin gave respective mean Rf values of 0.151 (values rangingbetween 0.12 and 0.19), 0.243 (0.20-0.30), and 0.382 (0.31-0.47). Thesefigures emphasize the necessity of simultaneously employing standards whereunknown samples are being determined, especially when controlled temperatureconditions do not prevail.
Two-dimensional Chromatography. It is seen in table 2 that severalof the Rf values fall within close range of each other. Compoundswhich group together in one-dimensional chromatographs can often be separatedby two-dimensional chromatography. With this technic a spot is placed nearthe corner of a square of filter paper and chromatographed in the usualmanner. After the solvent has run its course, the various compounds havealigned along one edge of the paper. When dry the paper is rotated 90 degreesand the spots rechromatographed with a different solvent, the new solventflows through the horizontal row of spots and transports them verticallyto another area of the paper commensurate with the Rf valueof the compound in that solvent. The completed sheet shows the spots distributedin characteristic positions throughout the paper. By plotting the Rfvalues of one solvent as abscissae against the Rf valuesof another solvent as ordinates, it is possible to approximate the positionsof spots as they would appear if these two solvents were used in a two-dimensionalchromatograph (19).
This has been done in figure 2 where isoamyl alcohol-acetic acid solventis used in the first phase of the two-dimensional scheme and isoamyl alcohol-ammunitionhydroxide solvent* is used in the second phase. The points placed alongthe abscissa and ordinate show the expected positions of the alkaloidshad they been developed in a one-dimensional system by the respective solvents.Experimental results closely approximate the calculated results shown diagramatically.While this two-dimensional system is not ideal (e. g., papaverine and narcotinestill remain unseparated), much of the crowding found in the one-dimensionalschemes is eliminated.
Chromatography of Alkaloids Extracted From Toxicologic Specimens.The final dry extracts obtained from tissues by the Stas-Otto procedureor from blood or urine by a new procedure developed in the 406th MedicalGeneral Laboratory and outlined in the section of this paper that dealswith toxicology, are transferred from 50 ml. beakers to 13 x 100 mm. liplesstest tubes (Wassermann type) using 2 ml. of boiling ethanol. The ethanolis removed by evaporation on
*This differs from the isoamyl-acetate acid solvent only in that concentrated ammonium hydroxide is substituted for glacial acetic acid.
189
a water bath. Three or four drops of ethanol are added to the tube and,while still in the water bath, the solution is drawn up and down a 0.1ml. Mohr pipette until a volume of 0.06 ml. is reached. Four 0.005 aliquotsof this are applied to the paper forming a single spot. Each aliquot isallowed to dry before making the next addition, thus keeping the area ofthe final spot at a minimum. The remaining two-thirds of the material issaved for chemical testing. Morphine, codeine, and heroin standards (freebases) serve as controls. A 0.005
FIGURE2. Diagrammatic representation of two-dimensionalpaper chromatograph of opium alkaloids.
ml. volume of 0.5 percent solution of each of the aforementioned alkaloidsis applied to a single point on the base line. The spots of the variousextracts to be tested are located at 3 cm. intervals on the base line,chromatographed in the manner previously described with the isoamyl alcohol-aceticacid solvent, and sprayed with the iodoplatinate reagent.
A comparison of the results obtained by chromatography and by chemicaltests (Marquis, Frohde, and Mecke) during the months of August, September,October, and November 1952, on a total of 221 cases is shown in table 3.
190
Table 3. Comparison of Chemical and Chromatographic Detection of Alkaloids
Chemical results | Chromatographic results | |
Negative | 173 | 146 |
Questionable | 20 | 30 |
Positive | 28 | 45 |
All but a small number of the cases in table 3 involve blood and urinespecimens. A good part of the discrepancy between the number of positivecases found by chemical and chromatographic methods results from the mannerin which the urine was processed. Gross and Thompson (17) foundthat hydrolysis* of urine from morphinized dogs increased the yield ofmorphine four or five times. This is due to the release of morphine froma combined form not recovered by usual extraction procedures. In orderto take advantage of this extra source of morphine, the hydrolysis procedurewas incorporated in the extraction process. A comparison of hydrolizedand unhydrolized urines soon verified the advantages of hydrolysis as wasevidenced by the greatly increased sizes and intensities of the developedmorphine spots. However, hydrolysis introduces a serious problem-that ofincreasing the impurities in the final extracts. These impurities are darkbrown and mask the chemical color reactions to the extent that, even whilegreater amounts of morphine are present, a more positive conclusion couldhave been reached had the urine not been hydrolized. These impurities donot present any difficulties in performing the chromatographic processsince they do not affect the progress of morphine migration and they riseto positions on the paper high above the morphine spot area.
The "questionable" cases of table 3 require some discussion.Almost all of those so classified from chemical tests were listed as positivechromatographically. On the other hand, those cases which were considered"questionable" chromatographically result from chromatographswhere only very weak morphine spots were observed, where morphine spotswere "off color," or where the Rf values were slightlydifferent from that expected for morphine. The very weak spots representquantities of morphine less than 10 micrograms. Chemical confirmation ofsuch minute amounts of morphine cannot be expected when it is rememberedthat the remaining two-thirds of unchromatographed extract must be dividedto make the several chemical tests.
*A suitable aliquot of urine was hydrolized with 10 percent by volume of concentrated hydrochloric acid in the autoclave by heating at 15 pounds pressure for 30 minutes.
191
The ideal chromatographic procedure would be specific for opium alkaloidsand nothing else. The achievement of such an ideal is not likely becauseof the non-specificity of alkaloid isolation methods, Rf valuesand spot detection reagents. That the procedure developed here is not idealis evidenced by the appearance of occasional miscellaneous spots. The distributionof the extraneous spots from extracts of blood, urine, stomach contents,liver, and brain from 201 negative cases is given in table 4.
Table 4. Distribution of Extraneous ChromatographicSpots
|
|
| Cases where miscellaneous spots were found | Percent of cases showing miscellaneous spots |
Blood | 152 | 7 | 5 |
Urine* | 86 | 18 | 21 |
Stomach Content | 20 | 10 | 50 |
Liver | 19 | 8 | 42 |
Brain | 10 | 3 | 33 |
*Includes both hydrolized and unhydrolized samples.
The high incidence of extraneous spots found in stomach contents extractsis to be expected since a large variety of alkaloids are found in the plantfoods commonly ingested. Blood and urine extracts showed the fewest spots,not only in the number of cases but in the number of spots per case. Undoubtedlysome of these unidentified spots represent alkaloids of pharmacologic interest.Fortunately, few of these spots fall in the morphine area and, when theydo, they show a violet coloration rather than the characteristic dark blueof morphine. These extraneous spots are quite often diffuse and leave trailingstreaks in their wake. They are easily recognized by an experienced technician.
Of particular interest are those extraneous spots found in positivemorphine extracts. In the sodium hydroxide-chloroform extract of most urinesamples showing a strong morphine spot, a second spot is found in the codeinerange. The persistent appearance of this spot in association with morphinesuggests that it represents a metabolic end product of this drug possiblycodeine. The conversion of codeine to morphine in tissues has been demonstrated(18); that the reverse process might take place is not a fancifulsuggestion. Quite often a third spot is found located between the codeineand heroin areas. A fourth spot is sometimes observed in the range of Rf60 to Rf 70. This spot is most commonly seen in hydrolized specimens.Attempts at identification of these materials are being made with the hopethat
192
something may be learned about morphine metabolism. There is some evidencethat addicts metabolize morphine in a different manner than novices (21,22), and it is possible that the appearance of the spots associatedwith morphine in some cases and not in others may reflect the degree ofaddiction of the subject. An alternative explanation may be that becausethe urine samples are collected at different times after the drug is administeredthey represent various stages of morphine metabolism.
While heroin is undoubtedly the offending drug in most subjects, thisalkaloid has never been observed on the chromatographs, thus supportingthe view that heroin is rapidly converted to morphine in the tissues.
These results show the validity of applying the paper partition chromatographictechnic to toxicologic problems. They also point out the need for an improvedmethod of purifying extracts.
Chromatographic Purification of Alkaloids Extracted from ToxicologicSpecimens. After establishing the presence of suspected opium alkaloidsby chromatography using one-third of the final extracts, the remainingtwo-thirds of the material is purified chromatographically before attemptinga chemical identification (when very large spots are observed on the preliminarychromatograph, only half of the remaining material is used for purification).Instead of spraying this second chromatograph, small squares of paper arecut from areas corresponding to the spot areas found on the preliminarysheet. These areas are located by clipping out a strip of paper containingchromatographed standards for morphine, codeine and heroin, spraying thisstrip, and then replacing the strip to act as a guide. The areas to beextracted can be even more perfectly located by cutting a 1 mm. strip upthrough the center of the chromatographic path, spraying this and replacingit in the sheet. Areas on both sides of the color band on this narrow stripare removed for extraction. The squares of paper are rolled into a cylinderand placed in Wassermann-type test tubes. The paper is wetted with 2 dropsof concentrated ammonium hydroxide and 2 ml. of chloroform is added. Thechloroform is boiled for a few seconds in a water bath and the chloroformextract transferred in 1 ml. aliquots to a second test tube contained inthe water bath, allowing the first portion of chloroform to evaporate beforeadding the second. Spraying of these small pieces of paper after extractionshows the alkaloid has been removed. Similar extractions employing ethanolproved unsuccessful; substances interfering with the chemical tests areextracted with ethanol. As a check against the possibility of one spothaving "wandered" into the "lane" of an adjacent extract,the sheet from which the squares have been cut is sprayed with the iodoplatinatereagent. In the experience of this
193
laboratory such migrations have not occurred, but it is felt that thisprecaution must be taken to obviate legal criticism.
The residue remaining after the chloroform is removed is colorless andof such minute quantity that only by close inspection can it be seen atall. For chemical testing, 5 drops of ethanol are added to the tube andthe tube warmed briefly in a water bath. Aliquots of this are dried ona spot plate or microscope slide for testing. The resulting reactions arecomparable to those seen with tests on pure alkaloid standards; backgroundcolors and charring are absent; the colors persist and show the classicaltransformations.
The chromatographic purification of alkaloids has been applied routinelyin this laboratory only during the last 3 weeks of December 1952, and,while there have not been sufficient cases to establish the absolute validityof the method, the results obtained thus far have been highly encouraging.Spots have been graded as to size and density so that a spot which is justdiscernible receives a rating of 1/2plus, a dense spot with dimensions of about 11/2x 2 cm. is classified as 3 plus, and other spots between 1/2and 5 cm. are graded accordingly. It is estimated that a 1/2plus spot of morphine contains about 5 to 10 micrograms and a 3 plus spot50 to 75 micrograms of the alkaloid. The chromatographically purified extractswere given a grade derived from the preliminary chromatograph, thus adjustingfor the amount of extract used. Thirty-four urine extracts that showedpreliminary chromatograph spots in the morphine area, with grades rangingfrom 1/2 to 5 plus,were purified as described above. All but l of 2l purified residues witha 3 plus rating or greater gave positive chemical reactions. The singleexception was obtained from an extract which showed a violet spot ratherthan the characteristic blue of morphine. Five 21/2plus, three 2 plus, and two 11/2plus urine residues were all positive. One 1 plus urine residue was positive,another negative. A single 1/2plus urine residue gave negative results. A purified extract from brain(1 plus) showed good positive chemical reactions. A codeine spot (3 plus)from a stomach contents extract was purified chromatographically and identifiedchemically. The advantages of paper purification become obvious when thesefigures are compared with those given for unpurified extracts in table4.
More recently it has been found that morphine can be recovered directlyfrom the sprayed spot. When the paper is sprayed lightly, only a smallamount of the morphine combines with the platinate. This lightly sprayedspot is treated with ammonium hydroxide and extracted as previously described.The residue thus obtained is indistinguishable from that recovered froman unsprayed area. This modification of the procedure eliminates the preliminarychromatograph,
194
and saves not only time, but working material. In addition, it enablesperfect delineation of the spot to be extracted.
Paper chromatography has not been applied to quantitative analyses ofthe alkaloids in the 406th Medical General Laboratory, but the above studiesindicate that the technic could be readily adapted to such procedures.
References
1. Cori, C. F., Villiaume, E. L., and Cori, G. T.: J.Biol. Chem. 87 : 19, 1930.
2. Haggard, H. W., and Greenberg, L. A.: J. Pharmacol.52 : 167, 1934.
3. Harger, R. N., Hulpiew, H. R., and Lamb, E. B.: J.Biol. Chem. 120 : 689, 1937.
4. Newman, H. W., Lehman, A. J., and Cutting, W. C.: J.Pharmacol. 61 : 58, 1937.
5. Harger, R. N., and Gross, A. J. L.: Am. J. Physiol.112 : 374, 1935.
6. Mirsky, I. A., and Nelson, N.: Am. J. Physiol. 127: 308, 1939.
7. Krantz, J. C., and Carr, C. J.: The Pharmacologic Principlesof Medical Practice, 2nd Edition, p. 420. Williams and Wilkins Company,Baltimore, 1951.
8. Ibid. 7, p. 431-432.
9. Newman, H. W., and Lehman, A. J.: J. Pharmacol 62: 301, 1938.
10. Ibid. 7, p. 557.
11. Williams, R. T.: Detoxication Mechanisms, Second Impression,pp. 214-222. John Wiley & Sons, Inc., New York.
12. Goldbaum, L. R.: J. Pharmacol. and Exper. Therap.94 : 66, 1948.
13. Loewe, S.: J. Pharmacol. and Exper. Therap. 84: 78, 1945.
14. Ibid. 7, p. 614.
15. Burger, A: Medicinal Chemistry, Vol. 1, pp. 157-159.Interscience Publishers, Inc., New York.
16. Clark, R. L., Pessolano, A. A., Weijlard, J., andPfister, K. S.: J. Am. Chem. Soc. 75 : 20, 1953.
17. Gross, E. G., and Thompson, F.: J. Pharmacol. &Exper. Therap. 68 : 413, 1940.
18. Block, R. J., Le Strange, R., and Zweig, G.: PaperChromatography. A Laboratory Manual, p. 4. Academic Press, Inc., New York,1952.
19. Consden, R., Gordon, A. H., and Martin, A. J. P.:Biochem. J. 38 : 230, 1944.
20. Adler, T. K., and Shaw, F. H.: J. Pharmacol. and Exper.Therap. 104 : 1-10. 1932.
21. Pierce, I. H., and Plant, O. H. J.: J. Pharmacol.and Exper. Therap. 46 : 201, 1932.
22. Zauder, H. D.: J. Pharmacol. and Exper. Therap. 104: 11-19, 1952.

